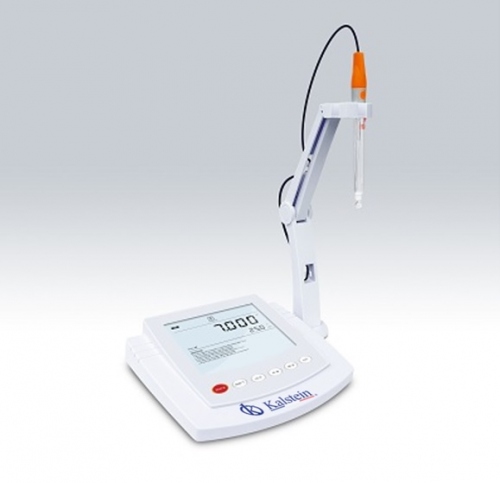
A pH meter is a laboratory equipment that is used to accurately measure the degree of acidity or alkalinity of a solution. This equipment provides information that expresses the degree of acidity of an acid or a base in terms of the activity of hydrogen ions.
The pH is the unit of measurement that describes the degree of acidity or alkalinity and is measured on a scale that goes from 0 to 14. The “p” is for potential, that is why pH is called hydrogen potential, and is expressed as the negative base 10 logarithm of the hydrogen ion concentration.
Electrodes of a pH meter
A pH meter is a specialized piece of equipment made up of three parts: a pH measuring electrode, a reference electrode and a high input impedance meter. The reference electrode has a known, constant and stable potential.
The electrode that measures pH is a glass bulb sensitive to hydrogen ions, with an output in millivolts that varies according to changes in the relative concentration of hydrogen ions inside and outside the bulb. The output of the reference electrode does not change with the activity of the hydrogen ions.
The pH electrode has a very high internal resistance, which makes it difficult to measure the voltage variation with pH. Therefore, the impedance of the pH meter input and leakage resistances are important factors.
How does a pH meter work?
The pH meter works by measuring the voltage between two electrodes, the electrode being an electrical conductor for non-metallic parts. Then it shows the value of the voltage transformed into pH levels.
Specifically, a pH meter works through an electrochemical cell mechanism, where the H + in the chemical reaction allow determining their concentration in solution. When determining the pH with a pH meter, a reference electrode and a measurement electrode are used, which are sensitive to analytes.
This is done thanks to its structure of two rods with electrodes, one made of calomel and the other made of glass. The sensor electrode is polarizable which gives it sensitivity to the concentration of hydrogen ions.
In other words, a pH meter is a high impedance amplifier that accurately measures the minimum electrode voltages and displays the results directly in pH units on an analog or digital display. In some cases, the voltages can also be interpreted for special applications or use with ion selective or oxidation / reduction potential electrodes.
At Kalstein we are MANUFACTURERS and we offer you excellent and innovative pH meters, designed with the highest quality and available at the best PRICES. That is why we invite you to take a look at: HERE