Spectrophotometers
Discover the power of accurate and reliable spectrophotometers for your laboratory needs. At Kalstein, we offer a wide range of spectrophotometers that cater to various applications, providing precise measurement of light intensity across different wavelengths. Our spectrophotometers ensure consistent and reproducible results, making them essential tools for research, quality control, and data analysis in the laboratory setting.
With user-friendly interfaces and advanced features, our spectrophotometers are designed to streamline your workflow and enhance efficiency. From UV-Vis spectrophotometers to fluorescence and infrared spectrophotometers, we have the perfect solution to meet your specific requirements. Trust in Kalstein for cutting-edge laboratory equipment that guarantees accuracy and reliability in every analysis. Elevate your research capabilities with our high-quality spectrophotometers today.

Types of Spectrophotometers Your Laboratory may need
Unique beam
Within the realm of Spectrophotometers, there exists a subcategory known as “Make it unique” (Haz único). This specific classification caters to laboratories seeking to stand out by customizing their equipment to suit their distinct requirements. From unique color combinations to personalized interfaces, the Haz único range offers a way to elevate your laboratory’s aesthetics while ensuring top-notch performance. With these specialized Spectrophotometers, you can enhance not only the functionality but also the overall look and feel of your laboratory space.
By opting for a Haz único Spectrophotometer, you are investing in more than just a piece of equipment; you are investing in a tailored solution that aligns perfectly with your laboratory’s needs and style. Whether you are looking to match your branding colors or simply want a device that reflects your laboratory’s individuality, the Haz único range provides a plethora of options to help you achieve a truly bespoke setup. Elevate your laboratory experience with a Spectrophotometer that is as unique as your research endeavors.
Double beam
Under the parent category of Spectrophotometers, the subcategory Double Beam offers a comprehensive range of double-beam spectrophotometers designed for accurate and precise laboratory measurements. These innovative instruments are equipped with advanced technology to ensure reliable results in various applications. The Haz Doble line combines efficiency with ease of use, making it ideal for research facilities, educational institutions, and industrial laboratories. With a focus on enhancing productivity and reducing errors, these spectrophotometers are a valuable asset for any scientific environment.
Kalstein’s Haz Doble collection features state-of-the-art models that deliver exceptional performance and durability. From UV-Vis spectrophotometers to fluorescence spectrometers, each instrument is crafted to meet the highest standards of quality and reliability. Whether analyzing samples in chemistry, biology, or environmental studies, Kalstein’s Haz Doble spectrophotometers provide accurate data for informed decision-making and research advancements.

SPECTROPHOTOMETER KALSTEIN
At Kalstein you can find the ideal Spectrophotometers for Your Laboratory

Micro-Spectrophotometer YR06034
YR06034 ultra-micro nucleic acid analyzer is an instrument used to detect the concentration and purity of DNA and RNA. The sample size required for each measurement is onl...
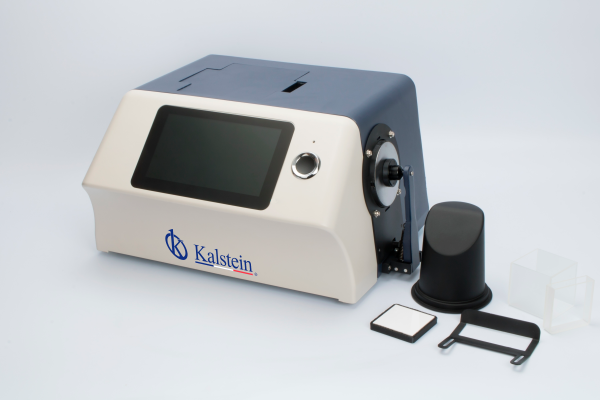
Benchtop Spectrophotometer YR05478
YR05478 desktop spectrophotometer can accurately measure and express various color difference formulas and color indexes in a variety of color spaces. The built-...
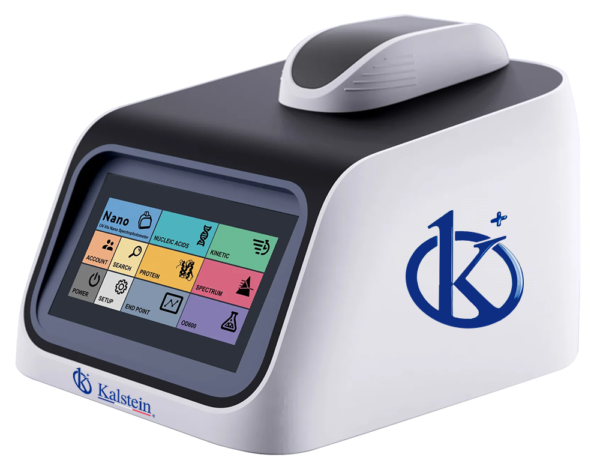
Biophotometer Micro Spectrophotometer YR06033
Smart and compact design, light and easy to move. 7 inches LCD color touch screen, stand-alone system and no need PC. User-friendly operating system, modu...
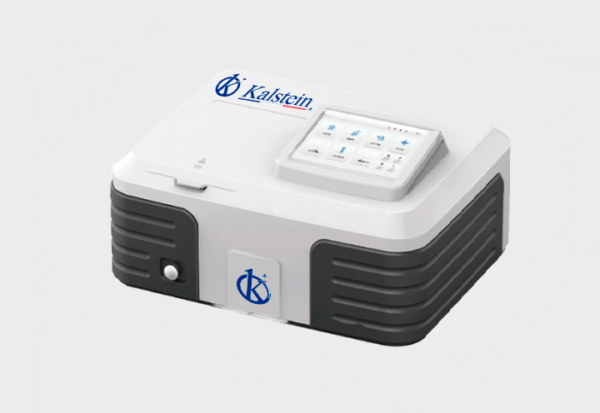
Spectrophotometer double beam YR01862-1 -YR01862-2
Full wavelength scanning (190-1100nm) covers ultraviolet, visible and near infrared regions. Fully sealed structure, all optical mirrors are equipped with Si...
Our Spectrophotometer best seller
YR05480 spectrophotometer is a color measuring instrument which adopts D/8 standard color measuring instrument (diffuse illumination, reception in 8°, SCI includes specular reflection light), non-contact test between test probe and tested sample, to obtain liquid and sauces. powder and other non-contact precision color measurement.
Features Of The Non-Contact Spectrophotometer
256-Pixel Dual-Matrix CMOS Image Sensor
Higher optical resolution ensures measurement speed, accuracy, stability and consistency of the instrument, mastering the core technology and achieving full compatibility on the same platform with international standards.
| Model | YR05480 |
| optical geometry | D/8 (diffuse illumination, reception at 8°, SCI includes specular reflection light); |
| Complies with CIE standard No.15, GB/T 3978, GB 2893, GB/T 18833, ISO7724-1, ASTM E1164, DIN5033 Teil7, GB 2893, GB/T 18833 | |
| Features | Non-contact test between the test probe and the tested sample, to achieve accurate non-contact color measurement of liquids, sauces, powders, etc. Mainly used for accurate color measurement and quality control of automated production lines; the measurement time can be adjusted from 0.2 to 1.5 seconds, and the software functions can be customized (additional customization function needs to be evaluated). |
| Light source | Full-spectrum LED light source, UV light source |
| Spectrophotometric mode | concave grid |
| Sensor | 256-pixel dual-matrix CMOS image sensor |
| Wave range | 400~700nm, 10nm output |
| Measured reflectance range | 0-200% |
| Measuring aperture | Φ20mm (Φ10mm can be customized) |
| Non-contact distance | 3.0 mm (±0.2 mm) |
| Sample height | Unlimited thickness, only test probes are used |
| Distance adjustment method | Fixed height according to actual sample |
| Measuring mode | Software customization function (additional customization function to be evaluated) |
| Locating | camera location |
| Color space | Laboratory CIE,XYZ,Yxy,LCh,LUV CIE,Musell,s-RGB,HunterLab,βxy,DIN Lab99 |
| Color difference formula | ΔE*ab,ΔE*94,ΔE*cmc(2:1),ΔE*cmc(1:1),ΔE*00,ΔE(Hunter),DINΔE99. |
| Other colorimetric index | WI (ASTM E313, CIE/ISO, AATCC, Hunter), |
| YI(ASTM D1925,ASTM 313), | |
| Index of metamerism MI, | |
| Stain resistance, color fastness, strength, opacity, opacity | |
| Observer angle | 2°/10° |
| Illuminant | D65,A,C,D50,D55,D75,F1,F2(CWF),F3,F4,F5,F6,F7(DLF),F8,F9,F10(TPL5),F11(TL84),F12(TL83/ U30) |
| Data shown | Spectrogram/values, sample chromaticity values, color difference/graphic values, color simulation, pass/fail result, display tolerance can be configured |
| Measuring time | As fast as 0.2 seconds |
| Data storage | Sample mode + Quality control mode 18,000 |
| Continuous statistical mode 30,000, for a total of not more than 48,000 | |
| Repeatability | In the optimal measurement mode (when the time of a single measurement is 1.5 seconds): |
| Spectral reflectance: within 0.1% of standard deviation: | |
| Chromaticity value: ΔE*ab within 0.03 (after warming up and calibrating the instrument, measure the average value of the slate 30 times within 5 s). | |
| Error between instruments | Within ΔE*ab 0.2 (Average value measured from 12 mosaics of BCRA series Ⅱ). |
| Method of measurement | Single measurement, average measurement (2~99 times) |
| Dimension | 200*200*160mm (test probe) |
| Weight | Approx. 3 kg (test probe only) |
| Energy | AC 24V, power adapter power supply 3A |
| Illuminating life | 5 years, more than 3 million times measurements |
| Monitor | 7-inch TFT color LCD, capacitive touch screen |
| Data port | USB, Bluetooth®5.0 |
| Language | Simplified Chinese, Traditional Chinese, English |
| Operating environment | 0~40 ℃, 0~85 % RH (non-condensing), altitude: less than 2000 m |
| Storage environment | -20~50℃, 0~85%RH (no condensation) |
| Standard accessory | Power adaptor, manual, data cable, standard calibration plate, black calibration box |
| notes | This model is especially suitable for optimized production lines, deep customization of functions will incur additional customization costs. |

Analysis of the best Spectrophotometers for Your Laboratory

Spectrophotometers: care and maintenance
A spectrophotometer is an instrument used in laboratories to measure the absorbance of a sample, as a function of the wavelength of an electromagnetic radiation, and thus to kn...

What is a Beam of Light in a Spectrophotometer?
The light beam of a spectrophotometer meets the projection of monochromatic luminescence to a sample, which is used to measure the amount of emission absorbed by said sampl...

Visible and UV Spectrophotometer: Differences
A spectrophotometer is an instrument that measures how much light a substance absorbs. This equipment measures the amount of absorbance after light passes through...

Spectrophotometer: kinetic curves
A spectrophotometer is a piece of equipment used to measure the absorbance of a sample, as a function of the wavelength of electromagnetic radiation. There are sever...
Catalog of models of Spectrophotometers on offer

Single Beam Visible Spectrophotometer – YR01844
Add to cart
Visible Spectrophotometer – YR01845
Add to cart
UV / VIS Spectrophotometer – YR01856
Add to cart
UV / VIS Spectrophotometer – YR01853
Add to cart
Visible Spectrophotometer – YR01847
Add to cart
Visible Spectrophotometer – YR01846
Add to cart
Spectrophotometer – YR01850
Add to cart
UV-VIS Ultraviolet Visible Spectrophotometer- YR01851
Add to cart
KALSTEIN UPDATED
Guidelines for you to become an expert in Spectrophotometers
The Spectrophotometers equipment are essential products in Your Laboratory, we provide you with guidance and recommendations for a better use, so you can work like an expert.
Fluorometer vs. Spectrophotometer
Spectrophotometry applies
How does a spectrophotometer work?
What technology uses a spectrophotometer?

Frequently asked questions from our customers about Spectrophotometers
The delivery time of your Kalstein product will depend on the following:
- Whether the equipment you are interested in is in stock or if it needs to be manufactured.
- The type of freight you have chosen, which can be either air or sea.
- Equipment in stock:
– Delivery Time (Air): 15-30 days.
– Delivery Time (Sea): 45-60 days.
- Equipment not in stock:
– Delivery Time (Air): 30-60 days.
– Delivery Time (Sea): 60-90 days.
You can make your purchase through:
- By email: [email protected]
- By phone: +33 (0) 1 70 39 26 50
- Online shopping: Through the official Kalstein website in your country.
At Kalstein, we provide our customers with inductions and technical support through new online methods. You can visit our induction videos, technical assistance, and guidance provided by a Kalstein team through our Youtube channel (Kalstein English). HERE
Send us a direct message and one of our agents will contact you
Spectrophotometers
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed dignissim placerat mauris cursus laoreet. Nam feugiat lacus ex, at fermentum sapien accumsan nec. Curabitur auctor porttitor mi non malesuada. Aenean condimentum, purus vitae rhoncus imperdiet, justo eros aliquam ipsum, at egestas leo diam eget libero.

Catalog of models of Spectrophotometers on offer.
-

Electric Heating Drying Oven YR06446
-

Electric Heating Drying Oven YR05259-2
-

Electric heating drying oven YR05248 // YR05255
This product has multiple variants. The options may be chosen on the product page -

Electric heating drying oven YR05244 // YR05247
This product has multiple variants. The options may be chosen on the product page -

Mini Centrifuge With Large Capacity YR012G
-

Tabletop High Speed Centrifuge YR0137-2 – YR0137-3
This product has multiple variants. The options may be chosen on the product page -

Gel Card Centrifuge YR142-3 – YR142-3-1
This product has multiple variants. The options may be chosen on the product page -

Tabletop High Speed Centrifuge YR019-TG
This product has multiple variants. The options may be chosen on the product page -

Intelligent Electric Wheelchair YR06432
-

Electric Wheelchair YR05445
-

Electric Wheelchair YR05443
-

Electric Wheelchair YR05442
-

Electric Wheelchair YR05444
-

Electric Wheelchair YR05441
-

Electric Wheelchair YR05440
-

Electric Wheelchair YR05439
Descubre más de nuestro catálogo
Tipos de Spectrophotometers

[Producto] A
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

[Producto] B
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Find out more about Spectrophotometers with our guides.
Medical Transport Stretchers: Safe and Efficient Mobility for Patients
Medical transport stretchers are a crucial tool in any hospital setting, ensuring the safe and comfortable mobility of patients. When...
Laboratory Consumables: Essential Materials for Uninterrupted Scientific Operations
Laboratory consumables are fundamental to ensuring the continuity of scientific research, as they are materials used regularly and indispensable in...
Lyophilizers: Drying Sensitive Samples Without Loss of Integrity
Drying sensitive samples is a critical task in many laboratories, especially when it comes to preserving the integrity of biological,...