Electrophoresis is an analytical method in which a controlled electric current is used to separate biomolecules according to their size-to-electric charge ratio, using a jellylike matrix as the base. When a mixture of ionized and net charged molecules are positioned in an electric field, they undergo an attraction force toward the pole with opposite charge, allowing time elapse the positively charged molecules move toward the cathode (negative pole) and those charged positively move toward the anode (positive pole).
Gel electrophoresis is used in laboratories to classify and measure protein or nucleic acids.
When did this technique start?
Electrophoresis began in earnest with the work of Arne Tiselius in 1931, and the new processes of separation and chemical analysis techniques based on electrophoresis continue to be developed in the 21st century. Tiselius, with the support of the Rockefeller Foundation, developed the “Tiselius apparatus” for motion limit electrophoresis. The method slowly extended until the advent of effective zone electrophoresis methods in the 1940s and 1950s, which use filter paper or gels as support for supporting media.
In the 1960s, gel electrophoresis methods became increasingly sophisticated, making it possible to separate biological molecules based on tiny physical and chemical differences, helping drive the rise of molecular biology. Gel electrophoresis and related techniques became the basis for a wide range of biochemical methods, such as protein fingerprinting, Southern hybridization and similar transfer procedures, DNA sequencing, and many more.
How does electrophoresis work?
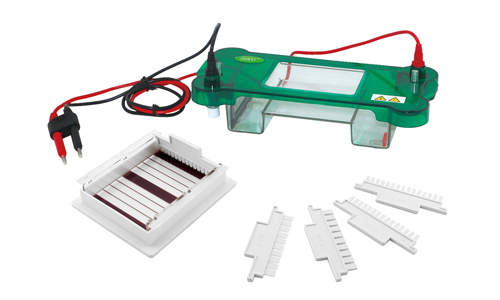
Electrophoresis equipment is an equipment that allows the separation of mixtures of particles with electrical charge in solution, taking advantage of the different migration speed when an electric field is applied (electrophoresis). It is also used for separation of DNA and proteins. With 4 outputs, the equipment has 4 levels of measurement: from 0 to 500 mA, from 0 to 400 V, and from 0 to 1 mA from 0 to 200 V.
Charged sample molecules will enter the gel through the planting well wall. Molecules with a negative charge migrate to the positive electrode (anode), and molecules with a positive charge migrate to the negative electrode (cathode).
The buffer serves as the conductor of electricity and controls pH, which is important for the stability of biological molecules. Because DNA has a negative charge at neutral pH it will migrate across the gel to the positive electrode during electrophoresis.
What considerations should you consider when applying this technique?
As with any scientific procedure, the possibility of human error and other sources of error exists and must be taken into account when interpreting the results. Considering the following: contamination of the sample Gel problems; incorrect sample loading, power current problems, and visualization problems.
At Kalstein we offer you a high quality system for your Electrophoresis runs. So we invite you to take a look HERE